The vehemence of the opposition to the move to lead-free solder is an indication that is arguably the most disruptive change that has occurred in the field of electronics assembly. It is disruptive because it requires a major change in a fundamental element of an electronics assembly: the connections between components. Until they are connected the components that make up a functional circuit are nothing more than a collection of materials subassemblies that by themselves can do nothing. In most cases the material that provides those connections also provides the assembly with the mechanical integrity it requires to survive the stresses and strains of the environment in which it has to operate. In this paper the authors will review the solder properties they consider important in a lead-free solder and report the results of measurement of these properties in a range of lead-free solders currently used or under consideration for commercial production.
Wave soldering is now taken for granted and sometimes considered a rather low technology process but its successful implementation required that the industry look more seriously at the soldering process and the factors that affect it than it had previously. The individual attention that could be applied to a joint when it was hand soldered by a person with a soldering iron was no longer possible. The era of mass soldering had begun and if the process was to deliver the expected economic benefits an acceptable solder fillet had to be achieved on all joints in the few seconds that they were in contact with the molten solder. If there had to be a team of operators “touching up” the joints on the board as it exited the wave soldering machine then the process had failed. A case can be made that it was the introduction of wave soldering that forced the electronics assembly business to start treating soldering as science-based technology rather than an artisan skill.
One response to this challenge was the development of the concept of solderability. Solderability is a measure of the ease with which a joint substrate can be wetted by molten solder and it continues to an important consideration in all soldering processes, particularly as the industry moves to lead-free. The industry responded to the challenge of mass soldering processes with the development of solderable finishes for components and printed circuit boards, fluxes that had high activity without corrosive residues and wave soldering machines with heating systems and wave dynamics that facilitated the process of forming joints that complied with quality standards.
In the context of the subject matter of this paper, it is worth noting at this point that one of the particular responses to the introduction of wave soldering was a change in the composition of the solder used in this process from the 60/40 tin-lead that had been used since Roman times to 63/37 eutectic. Perhaps for the first time a rather esoteric metallurgical term, “eutectic”, found its way into the vocabulary of electronics assembly. The availability of cheap mass-produced electronics made possible by wave soldering was undoubtedly one of the factors that led to the massive expansion in the application of electronics to all aspects of modern life that in turn has driven the development of other soldering methods such as reflow soldering.
When the pressure to increase the density of electronic circuitry as well a reduce cost led to the introduction of surface mount technology the challenge was so great that the electronics industry responded with a collective effort to cope with the change. The organisations formed at that time have remained to help the industry deal with subsequent challenges and are now active in helping the industry in its adoption of lead-free soldering. The introduction of a new soldering material, solder paste, required that those with the responsibility of managing electronics assembly had to cope with new concepts such as viscosity and thixotropic index, tackiness and open time. Machine makers were judged by the DT their ovens could deliver.
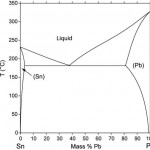
These foregoing changes were driven purely by technical and economic considerations. The Montreal Protocol provided the electronics industry with its first experience of a change that was driven by a concern about the environment. The fairly simple cleaning processes made possible by the great dissolving power of the chloro-fluorinated hydrocarbons that the industry was forced to give up had made it possible to use high activity high solids fluxes that provided a wide process window. Cleaning processes that match the effectiveness of the CFC solvents have subsequently been developed but the first response was to develop new flux technologies and improve the solderability of joint substrates so that reliable soldering could be effected with minimal, safe residues that did not need cleaning. It has to be acknowledged, however, that all of the foregoing changes were made without changing the basic metallurgy of the alloy used in printed board assembly which was rooted firmly in the tin-lead alloy system with usually never less than 36 % lead (figure 1).
Sometimes advantage was taken of the steeply rising solidus line at the lead-rich end of the system to make a high melting point solder which was useful in component manufacture. Sometimes up to 2 % silver or 2 % copper was added either to suppress erosion of those elements from substrates or soldering tools or to strengthen the solder by the introduction of an intermetallic phase to the microstructure. However, the properties of the solder were determined primarily by the fact that the alloy consisted of two metallic phases, tin with up to around 2 % lead in solid solution and lead with up to about 20 % tin in solid solution. And in the eutectic alloy, nominally Sn-37Pb these two metallic phases are present in the form of a fine lamella structure of interleaved layers.
Benchmarking against tin-lead solder
Whether the tin-lead eutectic really is the best alloy for soldering electronic assemblies or whether it is just that electronic circuit design and electronics assembly processes developed within the constraints of its limitations this alloy has to be considered the benchmark against which candidate lead-free solders can be judged. When there was no reason to consider alternatives tin-lead solder was so taken for granted that its properties have not always been recognised, even by those who are now so keen to keep on using it. The only things that most lead-free solders have in common with tin-lead solders is that tin is the major ingredient and that they work by establishing a metallurgical bond with the substrate by reacting to form an intermetallic compound with the substrate metals. The differences in the fundamental metallurgy of tin-lead and lead-free solders are greater than the similarities.
Solder properties
The properties of solders can be considered to fall into two categories, those that affect their performance in soldering processes and those that affect the reliability in service of solder joints made with them. Included on the first category are melting point, wetting properties, fluidity, aggressiveness towards copper, aggressiveness towards soldering equipment and stability in the molten alloy. In the second category are strength and ductility, impact strength, microstructure, stability of microstructure/ intermetallic growth and reliability.
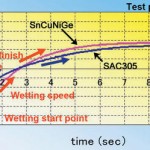
While in the early days of lead-free alloy development one of the primary objectives was getting a melting point as close as possible to that of Sn-37Pb it was found subsequently that less superheat (the extent to which the process temperature exceeds the melting point) is required with lead-free solders than with tin-lead solder. It was realised that the superheat in the tin-lead process was largely to get the joint to wetting temperature and preheat could achieve a similar effect. The temperature that it is necessary to reach to form a joint seems to be much the same for lead-free solders as tin-lead solder and this is probably related to the fact that in both cases the wetting reaction is the same; the joint achieves metallurgical integrity when the tin in the solder reacts with the substrate metal to form an interfacial intermetallic. An inference from this observation is that if it has other useful properties an alloy need not be eliminated from consideration because of a melting point that is higher but still within a range that components and circuit materials can tolerate. The standard wetting balance test can give a misleading impression of the effectiveness of an alloy to the extent that the wetting time includes the time taken to get the test piece to the melting point of the solder as well as the time that it takes for the solder to wet the substrate to the maximum extent. An alloy with a higher melting point can actually wet faster than one with a lower melting point. In figure 2 it can be seen that it takes a little longer for the test piece to get to the melting point of the SnCuNiGe but that alloy then wets it at a rate faster than that than of the lower melting point SnAgCu alloy. In practical soldering a difference in melting point can be compensated for by adjustments to other process parameters such as preheat. The more important property of the solder is its wetting rate.
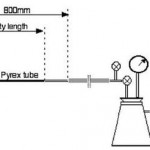
All soldering processes rely on the flow of solder into the joint. That process is driven by the capillary forces resulting from the wetting of the joint substrates but a property of molten metals known in the foundry industry as “fluidity” is also important. Like metal casting soldering relies on the flow of a molten metal that is close to its melting point to form the fillet and in wave soldering fluidity is also important in the drainage of excess solder that would otherwise cause shorts. A method developed by the foundry industry (figure 3) has been found to be useful in measuring the fluidity of solder alloy.
The significance of this property can be inferred from the fact that in the tin-lead alloy system fluidity is at its peak at the eutectic composition towards which the electronics industry migrated as the challenges that soldering processes had to deal with increased. Testing of lead-free solders currently being offered to the electronics industry indicate that fluidity measured at 40 °C above liquidus ranges from close to that of the tin-lead eutectic to 30 % lower. One of the unanticipated issues to emerge in the early stages of the implementation of lead-free soldering was the erosion of copper substrates. In figure 4 the solder flow is from left to right. Molten tin is quite aggressive towards most metals eroding them quite quickly. That aggressiveness is substantially reduced when the tin is diluted with lead so that dissolution of copper substrates by eutectic tin-lead solder has not been a major problem. Without that moderating influence lead-free solders tend to erode copper substrates so rapidly that the integrity of a solder joint to the copper foil of a printed circuit board can be compromised. There are significant differences between the rates at which lead-free solders erode copper.
Probably because of an interaction between silver oxide and the nickel and chromium oxides that make stainless steel “stainless” lead-free solders that contain silver, even at low levels, tend to erode soldering equipment. That tendency is exacerbated by the phosphorus that is usually also added to silver-containing lead-free solders to manage the high dross rate of these alloys. The erosion of the wave former in figure 5 occurred in less than a year’s normal operation with a low-silver SAC alloy. The erosion problem can be managed by the use of special materials, coatings and surface treatments but this add cost and does not necessarily provide a permanent solution. Silver-free solders with alternative anti-drossing additions can be used safely with plain stainless steel equipment. The new generation of “microalloyed” solder based around the tin-copper eutectic rely on additions of high melting point elements such as nickel and cobalt. For stable long term operation in solder baths it is essential that high melting point intermetallic phases do not form in the working solder bath if consistent soldering performance is to be maintained. The cubic cobalt-tin intermetallic that is stable at soldering temperatures settles out of the melt so that the microalloying addition is not available to modify the alloy behaviour as intended.
Start with the 2nd category
While tin-lead solder is a mixture of tin and lead metallic phases that are soft the intermetallic phases in lead-free solders are hard. Depending on their morphology and distribution the intermetallic phases can increase the yield strength of the solder. While the combination of intermetallics in SnAgCu alloys increase strength they also reduce ductility. While in some cases that higher strength is an advantage, the associated brittleness is the cause of many of the reliability problems that are being encountered with lead-free solders. One of the under-appreciated advantages of tin-lead solder was its ductility, i.e. its ability to absorb substantial strain without work hardening and cracking. The wide range of materials with different coefficients of thermal expansion that are used in the construction of electronics assemblies mean that there is substantial relative movement within the assembly. As the materials that hold these assemblies of different materials together the solder joints have to accommodate those strains. If the strain can be taken up by flexing of circuit elements such as component leads then the high yield point of some lead-free solders can mean a long service life for the solder. However, if the solder itself has to accommodate the strain then the rapid work hardening that occurs can result in early cracking and joint failure. Lead-free solders that do not contain silver have a lower strength but their higher ductility means that they can accommodate more strain without cracking. The analysis of reliability data confirms the benefit of alloy ductility when the solder joint is subjected to large accumulated strain.
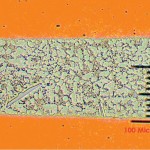
The use of electronics in portable devices that can be dropped has increased the likelihood of solder joints being subjected to impact loading. Testing of BGA at shear speeds of up to 4000 mm/sec reveals differences between lead-free solders in the sensitivity of their strength and fracture energy to shear speed. The tendency to low energy (brittle) fracture at high shear speeds in SnAgCu alloy is a consequence of brittle interfacial intermetallic and the high strain rate sensitivity of shear stress of the bulk alloy. While there was seldom a need to consider the microstructure of tin-lead solder the microstructure of lead-free solders is an important consideration in their performance in soldering processes and their reliability in service. If the only alloying additions are silver and/or copper then the matrix is nearly pure tin with the alloying additions present only in the form of intermetallic compounds with tin, Ag 3 Sn and Cu 6 Sn 5. While both phases in tin-lead solder are soft, ductile and metallic in character with a non-faceted morphology the intermetallic compounds in lead-free solders are hard, brittle and non-metallic in character. If the composition and cooling conditions favour their growth as the first phase then the intermetallic compounds have a distinctive faceted morphology (figure 6). Ag 3 Sn crystals take the form of flat plates while Cu 6 Sn 5 usually occurs as hexagonal prisms that are sometimes hollow. Even if the composition is nominally eutectic, such as the Sn-3.8Ag-0.7Cu alloy, unless the alloy has been modified by a further addition the difficulty of nucleating the intermetallic phases means that the alloy can cool to nearly 20 °C below its nominal melting point before solidification begins. This undercooling favours the growth of tin dendrites that can take up a major part of the microstructure before the resulting concentration of solute elements begins to favour solidification as a pseudo-binary or ternary eutectic with the characteristic fine dispersion of phases (figure 7). On the basis that the fine microstructure of the tin-lead eutectic free of primary tin dendrites is a desirable feature in a solder the fine dispersed microstructure that can be obtained by ternary additions to the tin-copper eutectic (figure 8) would be expected to enhance the properties of the alloy and there is some qualitative evidence to support that position.
The fine eutectic structure of tin-lead solder coarsens as the solder is aged at elevated temperature. And while there is some reaction between the tin and a copper substrate the rate of reaction is slowed by the dilution with lead and there is no other element playing a role in the process. The intermetallic phases in lead-free solders are more stable but the high tin content means that there is a tendency to more intermetallic growth at the interface. While silver seems to accelerate growth of the interfacial intermetallic nickel has been found to inhibit the growth of that intermetallic in the tin-copper eutectic system. Although initially thicker the intermetallic in the SnCuNi system grows more slowly than that in other alloy systems. The stability of the intermetallic is expected to have beneficial consequences for the reliability of this alloy in service.
Choosing a lead-free solder
The following considerations emerge from the first eight years of commercial mass production with lead-free solders and the testing and development that has been done to support this change in one of key materials of electronics assembly. For applications where a solder joint is likely to be subjected to substantial accumulated strain or impact loading choose a solder with good ductility and a stable interfacial intermetallic. Where the joint experiences Joule heating from carrying high currents the stability of the intermetallic at the interface and in the body of the solder has been found to be particularly important. Fluidity is a property that affects the performance of a solder in all applications and is a valid selection criterion. Fluidity is associated with a eutectic mode of solidification and the resultant fine uniform structure largely free of primary tin dendrites seems to be beneficial to the performance of an alloy as a solder.
EPP Europe 418
zusammenfassung
Die Autoren geben einen Überblick über die Lötmerkmale, die sie in einem bleifreien Lötmittel für wichtig erachten und sie berichten über Messergebnisse dieser Eigenschaften bei einer Auswahl an bleifreien Loten, die momentan eingesetzt werden oder für den Einsatz in der gewerblichen Produktion zur Diskussion stehen.
Les auteurs dressent un aperçu des caractéristiques de brasage qu’ils considèrent comme importantes dans un produit de brasage sans plomb. Ils citent également des résultats de mesure de ces propriétés dans une sélection de brasures sans plomb actuellement utilisées envisagées pour une utilisation dans la production industrielle.
Gli autori forniscono una panoramica sulle caratteristiche di saldatura che considerano importanti in una lega per saldatura senza piombo e riferiscono circa i risultati di misurazione di queste proprietà in una scelta di saldature senza piombo attualmente impiegate o che sono prese in esame nella produzione industriale.
Acknowledgements
Thanks are due to the following people who have contributed data included in this paper or who have made important contributions to the development of the authors’ views on lead-free solders. Iver Anderson and Jason Walleser- Iowa State University. Arne Dahle, Kazuhiro Nogita, Chris Gourlay, Tina Ventura, Jonathan Read, Bastian Meylan- University of Queensland. Craig Hillman, Nathan Blattau, Joelle Arnold- DfR Solutions, Shoichi Suenaga, Sonoko Seki, Masuo Koshi- Nihon Superior Co., Ltd. Jean-Paul Clech. Ron Gow-DKL Metals. Thanks are due also to the chairman of Nihon Superior Co., Ltd., Toshiro Nishimura for his continuing support for the company’s research and development programs.
Share: